100% Synthetic vs Full Synthetic
Before 1998 the term “100% Synthetic” meant that the base oils by regulation had to be Group 4 and 5 “Synthetic” Base Oil.
This is because Groups 1, 2 & 3 were classified as “Conventional” Mineral Base Oil.
Over 50 years ago AMSOIL was the First to develop an API-rated 100% Synthetic motor oil in 1972 using Group 4 and 5 “Synthetic” Base Oil.
After 1998 oil manufactures were allowed to call there oil 100% Synthetic or Fully Synthetic because now they mean the same thing… Why is that?
In 1998, Mobil confronted Castrol after figuring out that Castrol was refining “Conventional” (Group 3) Mineral Base Oil and marketing it as “Synthetic”.
The term “Fully Synthetic” was born after Castrol won the lawsuit. This allowed “Conventional” (Group 3) Mineral Base Oil to classified as Synthetic!
Fully Synthetic is a marketing term that in no way defines the amount (%) of genuine group 4 and 5 synthetic content in the oil bottle! It could be 90% Group 3 and 10% Group 4 or 5. Since Group 3 “Mineral” base oil is cheaper compared to Group 4 and 5 “Synthetic” base oil, There’s a massive cost savings for the oil manufactures when they can use more Group 3 Mineral base oil and still classify it as “Full Synthetic”. The problem is we will never know how much “Genuine Group 4 and 5 Synthetic” oil is actually in full synthetic motor oil since oil companies protect that information as proprietary…
While competitors have been formulating there oils down to a price AMSOIL has been formulating up to the highest standards. Don’t accept substitutes.
Today AMSOIL still produces there 100% Synthetic Lubricants using top tier Group 4 and 5 Synthetic Base Oil’s just like they did from the very beginning since 1972!
AMSOIL The First In Synthetics since 1972.
AMSOIL 100% Synthetic means EVERY SINGLE DROP of oil in your bottle is chemically engineered to form pure lubricants with predictable properties. AMSOIL 100% Synthetic Motor oils DO NOT contain conventional mineral oil or paraffins (wax).
AMSOIL motor oils are formulated using molecularly uniform synthetic base oils that slip easily over one another and are free of unwanted materials. Their versatility and pure, uniform molecular structures impart properties that provide better friction-reduction, optimum fuel efficiency, maximum film strength, and extreme-temperature performance conventional lubricants just can’t touch. AMSOIL lubricants help reduce energy lost to friction, maximize fuel economy which helps reduce greenhouse gases (CO2) emissions. AMSOIL offers superior wear protection in extreme temperatures. AMSOIL keeps engines 81% cleaner² (5 times cleaner) than conventional oil by better resisting the formation of deposits.
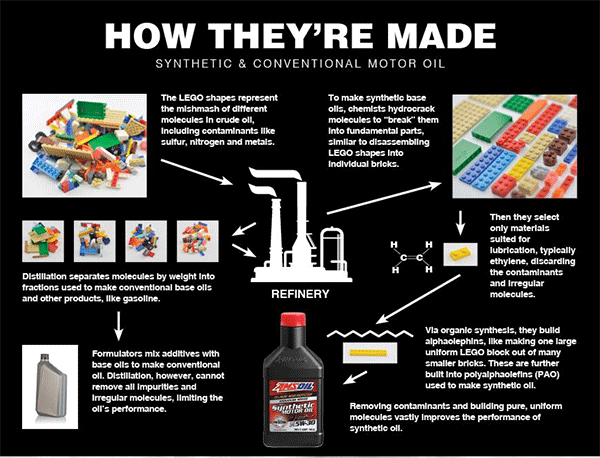
Synthetics are built, not distilled
Unlike their conventional counterparts, synthetic oils are “built,” not distilled. This means formulators start with individual molecules, typically ethylene if formulating polyalphaolefin (PAO)-based synthetic oil, and build the lubricant from the ground up in the laboratory.
To illustrate, think of crude oil like a pile of LEGO blocks haphazardly connected to form various shapes of different sizes. Each block represents a different molecule, including elements such as carbon, sulfur, nitrogen or oxygen.
Distillation separates the blocks into piles based on size. Larger blocks form a pile, medium blocks form another pile and so on. The fraction containing smaller, lighter molecules is used to make products like kerosene and gasoline. Larger molecules become tar. Medium molecules become products that include base oils.
Problem: Distillation cannot prevent irregular molecules or molecules unsuited for lubrication from contaminating the fraction intended for lubricating oils, reducing the finished product’s performance.
The process used to make synthetic oil solves this problem by removing contaminants.
Formulators start with a crude-oil fraction, or a pile of LEGO blocks. They use different chemical processes to “crack” the blocks into individual LEGO bricks, deconstructing each larger molecule into its constituent parts. They’re left with different molecules, like LEGO bricks spread out on a table.
They select only the pure, uniform materials best suited for lubricating an engine, which is typically ethylene when manufacturing synthetic lubricants. Using organic synthesis, chemists use ethylene to build larger molecules, called alphaolefins. Then they use alphaolefins to build polyalphaolephins (PAO). “Poly” simply means “many.” The final product is a PAO synthetic base oil used to make synthetic motor oil.
By building the finished product from only pure, uniform molecules, synthetic oils remain fluid in sub-zero cold for easier starts and better startup protection, resist evaporation in extreme heat, provide better wear protection and last longer.
The base oils are primarily responsible for fighting wear, reducing heat and minimizing friction. The addition of different chemical additives to the formulation enhances motor oil performance. Additives combat chemical breakdown, neutralize acids, provide additional wear protection and more, depending on the formulation and application for which its intended.
To illustrate, think of a quart of oil like a glass of lemonade. The base oils are the water and the additives are the lemon concentrate and sugar.
What are the different base oil groups?
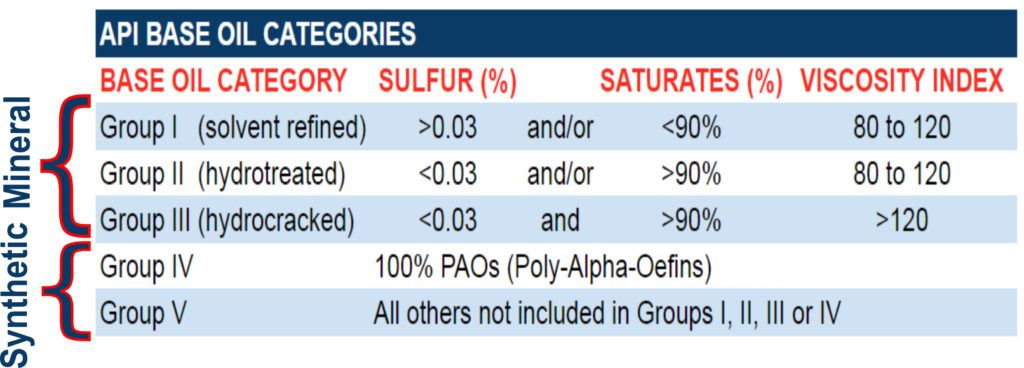
The American Petroleum Institute (API) developed a classification system for base oils that focuses on the paraffin and sulfur content and degree of saturation of the oil. These classifications are referred to as the base oil groups. The saturate level indicates the level of molecules completely saturated with hydrogen bonds, leaving them inherently un-reactive.
In simpler terms: Base Oil Groups are more resilient to chemical degradation, which means they last longer and perform better.
While base oils are a fundamental element in formulating motor oil and determining its synthetic content, they’re only part of the picture. Additives make up the other part of the equation, and the quality and concentration of additives have a significant affect on the oil’s ability to protect.
Look for motor oils that offer performance claims backed by industry-standard testing or real-world results. That’s what’s really important.
In essence, look for an oil that offers good overall protection, not just one that’s formulated with a specific type of base oil. A good way to identify a high-quality synthetic is to look for quantifiable performance claims. For example, we advertise AMSOIL Signature Series Synthetic Motor Oil‘s excellent wear protection, as proven in a real-world test. It offers 75 percent more engine protection against horsepower loss and wear than required by a leading industry standard.*
API Base Oil Categories
(Group I) (Mineral) (Solvent Refined) base oils are the least refined of all the groups. They are usually a mix of different hydrocarbon chains with little uniformity. While some automotive oils use these oils, they are generally used in less-demanding applications.
(Group II) (Mineral) (Hydrotreated Petroleum oil) are refined from crude oil. Contaminating elements such as sulfur, nitrogen, oxygen and metal components such as nickel or vanadium are inherent to crude oil and cannot be completely removed through the refining process. The oil refining process separates the various types of molecules in the oil by weight, leaving molecules similar in weight but dissimilar in structure, reducing performance.
(Group III) (Mineral) (Hydrocracked Petroleum Oil) is produced by hydrocracking crude oil. These oils are refined even more than Group II base oils. The result is a synthetic base oil free from the contaminants inherent to conventional oils. The purpose of this additional hydrocracking procedure is to produce a purer base oil from crude oil. These synthesized materials can be used in the production of synthetic and semi-synthetic “Fully Synthetic” lubricants.
(Group IV) (Synthetic) base oils are Polyalphaolefins (PAOs). They are chemically engineered to form pure lubricants with predictable properties. They contain no contaminants or molecules that don’t serve a designed purpose. Their versatility and pure, uniform molecular structures impart properties that provide better friction-reduction, optimum fuel efficiency, maximum film strength, and extreme-temperature performance conventional lubricants just can’t touch.
(Group V) (Synthetic) base oils are also chemically engineered oils that do not fall into any of the categories previously mentioned. Typical examples of Group V oils are esters, polyglycols and silicone. As with Group IV oils, Group V oils tend to offer performance advantages over Groups I – III. An example of a mineral-based Group V exception is a white oil, a very pure lubricant used in industries ranging from cosmetics to food processing. Some Group V oils are completely unsuitable for automotive use.
Summary
There are wide performance differences between base oil group categories. Generally speaking, Group IV base oils offer the best performance, Group III second best, and so on in reverse order. There are exceptions. You can’t judge motor oil performance completely on base oil type. You must take into account its entire formulation, including the additives.

